Some basic concepts of chemestry - Anjali sharma
Some basic concepts of chemistry
Significant figure
- All non-zero digits are considered significant. For example, 91 has two significant figures (9 and 1), while 123.45 has five significant figures (1, 2, 3, 4, and 5).
- Zeros appearing between two non-zero digits (trapped zeros) are significant. Example: 101.12 has five significant figures: 1, 0, 1, 1, and 2.
- Leading zeros (zeros before non-zero numbers) are not significant. For example, 0.00052 has two significant figures: 5 and 2.
- Trailing zeros (zeros after non-zero numbers) in a number without a decimal are generally not significant (see below for more details). For example, 400 has only one significant figure (4). The trailing zeros do not count as significant.
- Trailing zeros in a number containing a decimal point are significant. For example, 12.2300 has six significant figures: 1, 2, 2, 3, 0, and 0. The number 0.000122300 still has only six significant figures (the zeros before the 1 are not significant). In addition, 120.00 has five significant figures since it has three trailing zeros. This convention clarifies the precision of such numbers. For example, if a measurement that is precise to four decimal places (0.0001) is given as 12.23, then the measurement might be understood as having only two decimal places of precision available. Stating the result as 12.2300 makes it clear that the measurement is precise to four decimal places (in this case, six significant figures).
- The number 0 has one significant figure. Therefore, any zeros after the decimal point are also significant. Example: 0.00 has three significant figures.
- Any numbers in scientific notation are considered significant. For example, 4.300 x 10-4 has 4 significant figures.
Scientific notation
A method for expressing a given quantity as a number having significant
digits necessary for a specified degree of accuracy, multiplied by 10 to the
appropriate power, as 1385.62 written as 1.386 × 10
Calculations involving scientific figures
(1) In addition and subtraction, the result is rounded off to the last common digit occurring furthest to the right in all components. For example, 100 (assume 3 significant figures) + 23.643 (5 significant figures) = 123.643, which should be rounded to 124 (3 significant figures).
(2) In multiplication and division, the result should be rounded off so as
to have the same number of significant figures as in the component with the
least number of significant figures. For example, 3.0 (2 significant figures
) 12.60 (4 significant figures) = 37.8000 which should be rounded off to 38
(2 significant figures).
Laws of chemical combination
Law of conservation of mass Established by french chemist Antoine Lavoisier.Statement:-
In all physical changes and chemical reactions,the total mass of the products
is equal to the total mass of reactants.
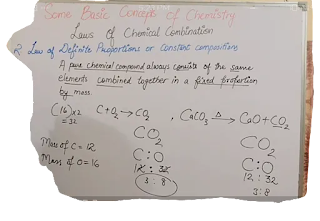
Laws of definite proportion or constant composition
A pure chemical compound always consists of the same elements combined together in a fixed proportion by mass.eg..water,carbon dioxide.Law of multiple proportions
if two elements combine to form two or more compounds,the mass of one of the element which combines with the fixed mass of the other in these compounds,bear a simple whole number ratio by mass.Law of reciprocal proportion
The ratio in which two compounds combine separately with a fixed mass of the
third element is either the same or simple whole no.multiple of the ratio in
which these elements combine with each other.
5.Gay Lussac Law of combining Volumes
Under similar condition of temperature and pressure,whenever gases
combine,they do in volume which bear a simple whole number ratio with each
other and also with the gaseous product.
Dalton’s Atomic Theory vs Modern Atomic Theory |
|
| Dalton’s atomic theory is a theory about indivisible particles called atoms which are the smallest particles of all matter. | Modern atomic theory is the theory that explains the fully detailed structure of an atom. |
| Structure of Atom | |
| According to Dalton’s atomic theory, atoms are indivisible particles. | Modern atomic theory says that atoms are composed of subatomic particles; protons, electrons, and neutrons. |
| Isotopes | |
| Dalton’s theory does not explain details about isotopes. It states that all atoms of the same element are identical. | Modern atomic theory explains details about isotopes having a different number of neutrons and the same number of protons. |
| Electrons | |
| Dalton couldn’t give details about electrons. | Modern atomic theory explains the location, reactions, and behavior of electrons. |
| Chemical Reactions | |
| Dalton’s atomic theory explains that atoms are the smallest particle that can be engaged in reactions. | Modern atomic theory states that subatomic particles can participate in reactions. |








Post a Comment